Courtney Kerestes
Pharmaceutical syntheses via cascade Diels-Alder reactions
Chemistry, Dr. Bur
About My Research:
I have spent this summer working on making a compound that is a precursor to many biologically active compounds. The purpose of my work is to find a simple pathway to a core bicyclic structure in order to simplify the synthesis of the biologically active compounds so they might be viable pharmaceuticals. Eventually this synthesis could be a part of the production of drugs with known cancer and tumor inhibiting properties. The basic synthesis proposed by Dr. Bur involves only a few steps that are done under inert conditions in one flask. Using cascade reactions like this one is a great way to cut down the reaction time and the excessive waste of petroleum-based solvents. Much of my work has been in optimizing the reaction so we can get a clean product with a decent yield. Once we figure out the right conditions in which to do this reaction with the starting materials we are currently working with, we will use slightly different compounds so we can get a variety of substitution in the product that would imitate the different substitutions necessary in the drugs.
This research was supported by the 2009 Merck-AAAS Summer Research Fund.
In this student's own words:
"I have never before had a job were I’ve been paid to learn and do something I love, so that in itself is a highlight. Also, research never feels contrived or pointless. I feel like my work is valuable, and even if I do not complete all of the work I would like to do on my project while I am at Gustavus, someone will continue it in the future until the goal has been achieved."
Professional Abstract:
The synthesis of a fused aromatic and lactone ring by way of a cascade Diels-Alder reaction was explored in order to replicate precursors of biologically active molecules. A siloxyfuran was deprotonated so the resulting carbanion could add to an aldehyde. Triethylsiloxyfuran was preferred to triisopropylsiloxyfuran because the large steric hindrance of the latter caused an elimination product to predominate. Maleic anhydride or another dienophile was attached to the oxide from the aldehyde so an intramolecular Diel’s-Alder reaction would occur. The Diels-Alder product, a tricyclic ring, broke down and lost H2O in order to become aromatic. The reaction is outlined in the scheme below. Adding substitution to the furan and using a variety of aldehydes and dienophiles could expand this scheme in the future.
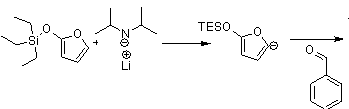
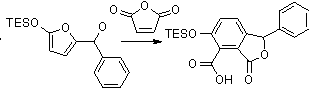
More student thoughts:
" Research with a Gustavus professor taught me that just because the professors are intelligent does not mean they are trying to intimidate you. More than anything, they are interesting in having you learn throughout the research. They understand from personal experience that research is unpredictable, so they would never criticize you for not getting the expected results. In the future, I will not hesitate to ask a teacher for help, now that I have gotten more comfortable with a few of them."
Links:
- Learn more about the Department of Chemistry
- Go to the student's Faculty Research Advisor