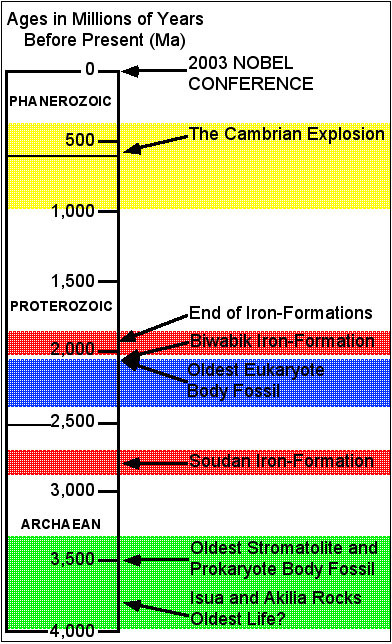
The early Earth atmosphere was a stinky cloud of carbon dioxide, methane, and hydrogen sulfide. So when did oxygen become integrated into the atmosphere? How did it occur? The evidence is both informative and lovely to look at and is found in northern Minnesota.
Iron can occur naturally in two states. Reduced, or ferric iron, is soluble in water. In the Archaean oceans, there must have been a great amount of ferric iron belched out from Earth’s interior or weathered from the continent. However, if any oxygen is present, the iron becomes oxidized to ferrous iron and precipitates out as a solid. The iron formations found in the Vermillion and Mesabi Iron Ranges, as well as other areas in the world, were formed from this process. The cyclical layering, or ‘banded-iron formation’ suggests a seasonal fluctuation in oxygen levels, though the time scale is unknown. Where did the oxygen come from? The deduction finger points to the lowly photosynthesizing bacteria that formed the stromatolites. Who would have thought that their waste product would be the ticket to the success of animals? What form of life will benefit from our pollution?
Samples of iron-formation include both high-grade natural ore from the Soudan area and low-grade taconite from Hoyt Lakes. Also, note the stromatolite from the Biwabik iron-formation (natural ore) and the banded iron-formation from Isua, Greenland.
Quick Links to: Introduction Oldest Rocks on Earth Oldest Fossils Prokaryotes vs. Eukaryotes Oldest Eukaryotes Oxygenation of the Atmosphere Ediacaran Fauna Snowball Earth Oldest Skeletons Pre-Cambrian/Cambrian Boundary Links for more information