 |
 |
Computational and Experimental study of isomerization in N,N-dimethylacetamide (DMA) and its derivatives
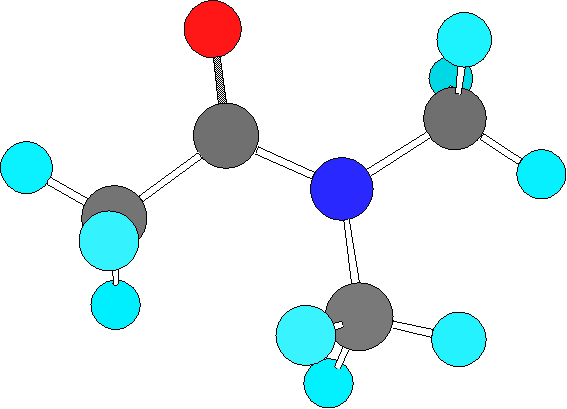
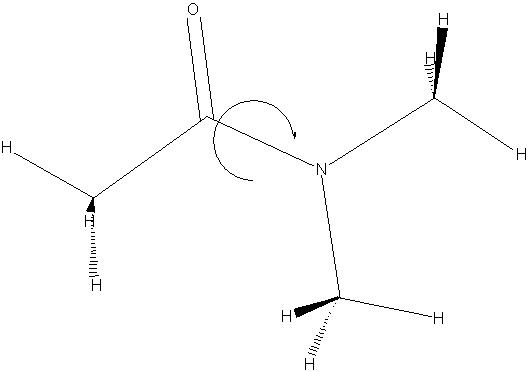
In this investigation, we explore the kinetics of the hindered rotation about the C—N bond in N,N-dimethylacetamide (DMA). If DMA were a completely rigid molecule, it is expected that the amino methyl protons will exhibit two resonances integrating for three each in the proton NMR, corresponding to CH3 groups that are "cis" and "trans" to the carbonyl group. If, on the other hand, there was free rotation about the C—N bond, then one would observe only one resonance as all six protons would have the same "average" chemical environment. In fact, DMA is an intermediate case, with two, slightly broadened resonances existing at room temperature. With increased temperature these resonances broaden and coalesce into a single transition. Our goal is to determine the activation barrier for the rotation about the C—N bond by measuring the rate of the isomerization as a function of temperature.
We will first study this system from a computational perspective trying to understand the relative energies of the different conformers and the electronic structure of these compounds. Our next investigation will be to examine the temperature dependent NMR spectrum to get a sense as to the relative population of these conformers and the barrier to isomerization.
In preparation we will read a paper to determine previous experimental and computational approaches to this question.
 |
 |
![]() Steps
Steps![]()
Resonance Interactions in Acyclic Systems. 3. Formamide Internal Rotation Revisited. Charge and Energy Redistribution along the C-N Bond Rotational PathwayKenneth B. Wiberg and Curt M. Breneman
Journal of the American Chemical Society, 114, 831-840 (1992).{copy available outside my office}Questions:
1. What do they find from their computations to be the barrier to isomerization?
2. Why is it useful to look at the changes in C-N and C-O bond length upon isomerization?
NMR Determination of the Rotational Barrier in N,N-dimethyl Acetamide
Francis P. Gasparro and Nancy H. Kolodny
Journal Of Chemical Education, 54, 258-261 (1977).{copy available outside my office}
Computational Investigation
Draw N,N-dimethylacetamide, formamide, and methyl-formamide in HyperChem
Optimize and examine these structures within two different model chemistries.
Compare the structures and record some pertinent geometrical data.
Examine the energy associated with the barrier to isomerization using the potential energy function in HyperChem
Examine the molecular orbitals and the charges on the atoms to gain insight into changes upon isomerization, the nature of the 90 degree (transition state structure).
Discuss in detail any conclusions that can be drawn from these calculations and compare them to the higher level calculations and results presented in the above paper..