Conformational
Flexibility of Tryptamine and 5-Hydroxytryptamine
Project Description
Purpose
There is a great deal of interest in determining the geometry and flexibility of the neurotransmitter serotonin (5-hydroxytryptamine) in solution. Serotonin is a biologically important molecule which plays a significant role in the regulation of mood, eating, and sleep as well as other functions.1. Discerning the basic molecular structure under biological conditions has important implications for understanding the binding behavior of serotonin to its receptors. It is thus my goal to use spectroscopic and computational means to study this system and its conformational flexibility. Specifically, I intend to measure the resonance Raman spectra of these molecules in aqueous solution.
|
|
|
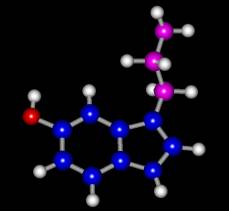
Figure
1. 5-hydroxytryptamine; Ethylamine sidechain is in purple. Tryptamine has the same structure less the hydroxyl group (in
red). |
In particular, the conformation of the ethylamine side-chain plays a role in its binding to receptors. As seen in Figure 1 the ethylamine sidechain (in purple) has a great deal of flexibility due to rotation about the single bonds. This flexibility leads to a number of stable conformations some of which may be important to the structure in aqueous solution. Resonance Raman spectroscopy provides an ideal way to probe this flexibility because it reveals important vibrational frequencies and their changes upon solvation. Detailed analysis of these frequencies can give insight into the molecular geometry. Gas-phase studies of the closely related tryptamine have revealed nine important conformations2. Which one or combination of these conformations is most stable in aqueous solution is unclear. It has been shown that carbon-hydrogen vibrational stretching frequencies are sensitive to orientation and have also been shown to be sensitive to solvent interaction.2. These vibrational frequencies can be determined with resonance Raman experiments and should occur with a Raman shift in the range of 2800 cm-1 to 3000 cm-1.
1. Bellec, N.; Boubekeur, K.; Carlier, R.; Hapiot,
P.; Lorcy, D.; Tallec, A. Journal of Physical Chemistry A 2000, ASAP.
2. Davy, R. D.; Schaefer, H. B. I. Journal of
Physical Chemistry A 1997, 101, 3135-3142.