AbsorptionSpectraofConjugatedDyes
![]()
In this investigation you will measure the absorption spectra of a series of cyanine dyes. Your experimental observations will be examined in the framework of a simple quantum mechanical model, the particle in a box model. Prior to the laboratory you should familiarize yourself with the structures of the molecules to be studied.
Experimental Method:
The dyes you will be using in this experiment are toxic. You should be careful in working with them, and avoid getting the solutions on your skin. In addition, these dyes will slowly degrade in the presence of light, so you should keep the solutions in the dark when they are not in use. The manufacturers claim 97% purity for the three dyes listed below. You should obtain spectra of the three cyanine dyes indicated in Table 1.
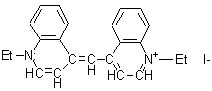 |
1,1 '-diethyl-4,4'-cyanine iodide
Table 1.
|
Dye |
M.W. (g mol-1) |
e(105M-l cm-l)a |
P |
|
1,1 '-diethyl-2,2'-cyanine iodide |
454.4 |
0.75 |
3 |
|
1,1'-diethyl-2,2'-carbocyanine iodide |
480.4 |
1.95 |
5 |
|
1,1 '-diethyl-2,2'-dicarbocyanine iodide |
506.4 |
2.13 |
7 |
aMolar absorption coefficient in methanol at the absorption maximum (from reference 2).
P = # of carbon atoms in chain of conjugation.
N = P + 3 = # of conjugated » electrons in cyanine chain
We will use methanol as the solvent. The molar absorptivities (extinction coefficients) of these dyes are quite high, so plan your dilutions to minimize the use of the solutes and solvent. Choose final concentrations such that A~0.5-1.0 for a standard, 1-cm pathlength cuvette. (Remember Beer's Law: A = ecl.). Record spectra against a solvent reference, scanning from 400-800 nm. Note the colors of the stock solutions and any changes in color that occur when the solutions are diluted. You will be using the Ocean Optics UV-Visible spectrometer to record the spectrum of each dye.
Draw these molecules in HyperChem and using a semiempirical CI calculation calculate the electronic spectrum. Place this spectrum in your notebook. What does the structure look like? Explore the molecular orbitals of this series of compounds.
Determine how the particle in a box formulation can explain the absorption spectra of these dyes in the free electron model. Develop a formula on this basis.
Analysis:
Your computer notebook should include a concise summary of the data, i.e., a table comparing your measurements of lmax with the lmax determined from the free electron model. In comparing experiment and theory, the wavelength of maximum absorption first should be calculated with a = 0, i.e., no adjustment for end effects. You then should fit the data to the best possible a, using a non-linear least squares fit within SigmaPlot (Regression Wizard- Equation Category – User-Defined). Record data on the quality of the fit as indicated by Sigma Plot. You also should analyze the lmax for the members of the 1,1'-diethyl-4,4'-cyanine iodide series listed in Table 2. (data obtained from Fisher and Hamer).
Table 2.
|
Dye |
lmax |
P |
|
1,1'-diethyl-4,4'-cyanine iodide |
593 |
7 |
|
1,1 '-diethyl-4,4'-carbocyanine iodide |
704 |
9 |
|
1,1'-diethyl-4,4'-dicarbocyanine iodide |
810 |
11 |
|
1,1 '-diethyl-4,4'-tricarbocyanine iodide |
932 |
13 |
P # of carbon atoms in chain of conjugation
N = P + 3 = # of conjugated ir electrons in cyanine chain
As with the 2,2' series, use the free electron model to calculate the lmax’s with a = 0 and with the best a determined by a non-linear least squares fit. (Do not despair if your a's lie somewhat outside the 0 <a< 1 range.)
As part of a brief discussion, compare the a's for the two series and try to explain any differences based on differences in the molecular structures. You also should comment on the appropriateness of the free electron/particle-in-a-box model for describing the spectra of the cyanine dyes. Your answer to this question should go beyond simply stating whether the model is "good" or "bad."
References:
1. N.I. Fisher and F.M. Hamer, Proc. Roy. Soc., Series A 154, 703 (1936).
2. S.E. Sheppard, and A.L. Geddes, J. Am. Chem. Soc. 66, 2003 (1944).
3. H. Kuhn, J. Chem. Phys. 17, 1198 (1949).
4. J.R. Platt, J. Chem. Phys. 22, 1448 (1954).
5. W. West, and S. Pearce, J. Phys. Chem. 69, 1894 (1965).